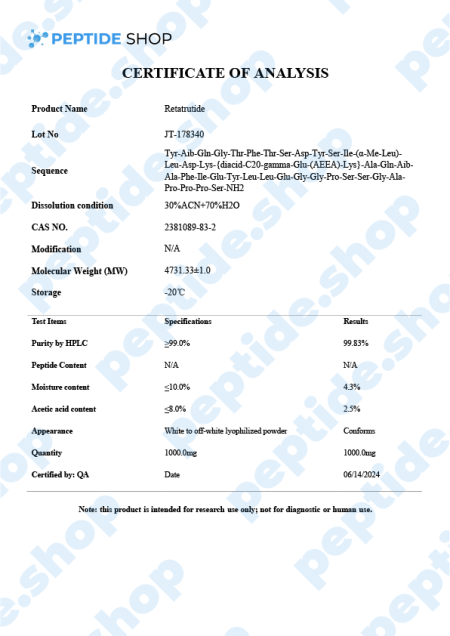
-
Retatrutide 5mg/10mg $150.00 – $270.00
Retatrutide is one of the latest incretin mimetic drugs being tested for their weight reduction properties. Researchers use incretin mimetic agents as they work to mimic incretin hormones (gut peptides that get secreted after we eat) to allow for a better insulin secretion and hyperglycaemic control.
Even though there are numerous weight reduction agents being prescribed and used, FDA-recognized “treatment” for nonsyndromic obesity are:
- Orlistat
- Phentermine-topiramate
- Naltrexone‐bupropion
- Liraglutide and
- Semaglutide
With the exception of glucagon-like peptide 1 receptor agonists (such as semaglutide), most weight reduction agents come with a specific set of side effects such as nausea, gastrointestinal distress, abdominal pain, vomiting, diarrhea, or a general weight decrease that does not cross the 15% mark. Some of these medications even come with a warning against using them if a patient is suffering from a kidney, heart, thyroid or any related problems.
This is why scientists are turning more and more to retatrutide, because this incretin mimetic decreases appetite, promotes the feeling of fullness and slows down the process of gastric emptying, but does all that while presenting with minimal side effects and increased weight loss effectiveness, compared to liraglutide or semaglutide.
Retatrutide Mechanism of Action
Retatrutide exerts a powerful glucagon-receptor agonistic effect. When we compare it to human glucagon and glucagon-like peptide 1 (GLP-1), it exhibits:
- Reduced potency on the GCGR (human glucagon receptor) and GLP-1R (glucagon-like peptide-1 receptor)
- Enhanced potency at the human GIPR (gastric inhibitory polypeptide receptor)
When talking about in vitro experiments, retatrutide demonstrated similar efficacy in evoking glucose production within hepatocytes and inducing lipolysis. Also, retatrutide showed a favorable half-life of 6 days, enabling it a weekly dosage regimen (should the drug get FDA approved).
Another double-blind study conducted in the US on obese patients showed promising results. 338 adults in total were involved, split into 6 cohorts (depending on the weekly dosage), up against a placebo group. Retatritude group experienced the following results:
- 1mg group – after 24 weeks this group experienced weight loss of 7.2%, and after 48 weeks a total loss of 8.7%
- 4mg group (commencing with 2mg) – after 24 weeks this group experienced a weight loss of 11.8%, and after 48 weeks a total loss of 16.3%
- 4mg group (commencing with 4mg) – after 24 weeks this group experienced a weight loss of 13.9%, and after 48 weeks a total loss of 17.8%
- 8mg group (commencing with 2mg) – after 24 weeks this group experienced a weight loss of 16.7%, and after 48 weeks a total loss of 21.7%
- 8mg group (commencing with 4mg) – after 24 weeks this group experienced a weight loss of 17.9%, and after 48 weeks a total loss of 23.9%
- 12mg group (commencing with 2mg) – after 24 weeks this group experienced a weight loss of 17.5%, and after 48 weeks a total loss of 24.2%
In contrast, the placebo group only experienced a 1.6% weight loss after 24 weeks and 2.1% after 48. Findings also showed that adverse effects related to retratitude treatment (such as nausea, vomiting, diarrhea and constipation), were mild and depended on the dosage.
In conclusion, this peptide showed promising results in addressing obesity in both in vitro and human test subject studies. However, test samples were relatively small, and we still need more research and clinical trials to show the effectiveness of retratutide as an effective, long-term way of managing weight.
Reference:
Naeem M, Imran L, Banatwala UESS. Unleashing the power of retatrutide: A possible triumph over obesity and overweight: A correspondence. Health Sci Rep. 2024 Feb 5;7(2):e1864. doi: 10.1002/hsr2.1864. PMID: 38323122; PMCID: PMC10844714.

Collection

the best price

& Winter 2018

 Anti Aging
Anti Aging Hair Growth
Hair Growth Muscle Growth
Muscle Growth Peptide Blends
Peptide Blends Peptide Supplies
Peptide Supplies Peptides
Peptides Skin
Skin Testosterone
Testosterone Weight Loss
Weight Loss