MOTS-C 10mg
Aging happens when cells within the body produce less energy and don’t function properly. The main part of the cell, mitochondria, is responsible for producing cellular energy. It also communicates with the cell’s nucleus, enabling the cell to function properly.
This communication happens through amino acid chains called MDPs or mitochondrially derived peptides. MDPs turn various functions on and off. The symptoms of aging seem to be related to genetic messaging. At the moment, scientists are working to identify each MDP. They have successfully identified one that appears to have multiple benefits when it comes to aging, called MOTS-c.
What is MOTS-c?
This peptide is made from 16 amino acids that manage communication between the nucleus of each cell and mitochondria. Some clinical trials suggest that MOTS-c may positively affect health and well-being, regulating how cells produce energy and control cellular function.
Benefits of MOTS-C
According to some test trials conducted in laboratory settings, scientists concluded the following benefits. However, more research is necessary to support these claims.
Improved Metabolism
Animal studies indicate that MOTS-c improves both glucose metabolism and insulin sensitivity. Therefore, this peptide could be a potential treatment for age-related metabolic conditions, like type 2 diabetes.
Mitochondrial Performance
Numerous age-related conditions and illnesses are linked to minimized mitochondrial function. However, this peptide optimizes mitochondrial function and might help reduce the incidence of disease and cellular damage.
Muscle Strength
As they age, people tend to lose muscle mass, leading to loss of balance, strength, and mobility. Studies conducted on animals indicate that MOTS-c could promote muscle endurance and muscle mass growth.
Stress Resilience
In response to stress, our body releases hormones such as adrenaline and cortisol. These hormones boost blood pressure and heart rates to help your body cope with whatever is causing stress. For the most part, this could be advantageous on a short-term basis.
However, if you expose your body to chronic stress for weeks, months, and years, it could lead to high blood pressure, type 2 diabetes, heart disease, and other stress-related illnesses. MOTS-c could help cells adapt to stressors, which in return may slow down cellular aging.
DNA Repair
Damaged DNA contributes to the aging process. In many studies, MOTS-c has been shown to support DNA repair. It may also positively affect autophagy, the process in which cells remove components that are no longer working or are damaged.
References:
Zheng Y, Wei Z, Wang T. MOTS-c: A promising mitochondrial-derived peptide for therapeutic exploitation. Front Endocrinol (Lausanne). 2023 Jan 25;14:1120533. doi: 10.3389/fendo.2023.1120533. PMID: 36761202; PMCID: PMC9905433.
Wan, W., Zhang, L., Lin, Y. et al. Mitochondria-derived peptide MOTS-c: effects and mechanisms related to stress, metabolism and aging. J Transl Med 21, 36 (2023). https://doi.org/10.1186/s12967-023-03885-2
Reynolds, J.C., Lai, R.W., Woodhead, J.S.T. et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat Commun 12, 470 (2021). https://doi.org/10.1038/s41467-020-20790-0
Zheng Y, Wei Z, Wang T. MOTS-c: A promising mitochondrial-derived peptide for therapeutic exploitation. Front Endocrinol (Lausanne). 2023 Jan 25;14:1120533. doi: 10.3389/fendo.2023.1120533. PMID: 36761202; PMCID: PMC9905433.
$110.00
| Quantity: | 10mg |
|---|---|
| Unit: | 1 vial |
| Contents: | MOTS-C |
| Form/Appearance: | Lyophilized/Powder |
| Peptide Purity: | 99% |
| Sequence: | Met-Arg-Trp-Gln-Glu-Met-Gly-Tyr-Ile-Phe-Tyr-Pro-Arg-Lys-Leu-Arg |
| Molecular Mass: | 2174.64 g/mol |
| Solubility: | Sterile / Bacteriostatic water |
| Synonyms: | Mitochondrial-derived peptide MOTS-c, Mitochondrial open reading frame of the 12S rRNA-c |
Based on 0 reviews
|
|
|
0% |
|
|
|
0% |
|
|
|
0% |
|
|
|
0% |
|
|
|
0% |
FAQs
Related Products
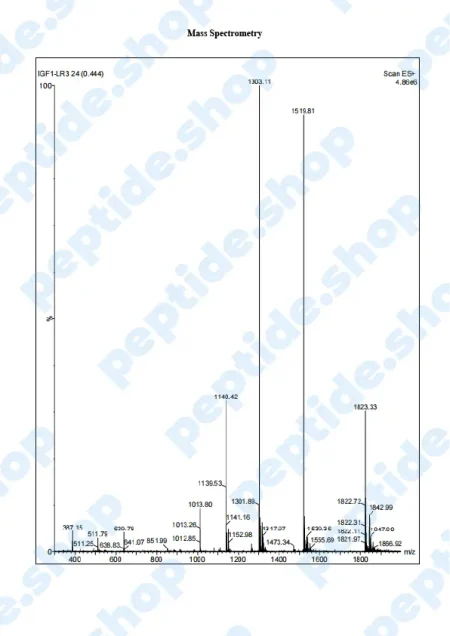
PeptideShop.com is selling insulin-like growth factor 1 (IGF-1) LR3 in vials of 3mg, in a lyophilized state, and it requires dissolution. Your choice of diluent will depend on the nature of your lab testing, but the most common ones are certainly sterile distilled water, or a sterile dilute acetic acid.
IGF-1 LR3 is based on IGF which has been chemically altered to have a prolonged half-life. This allows it to be more effective and increases the duration of its action.
IGF-1 is a 70 amino acid long peptide chain, similar in structure to insulin, which is able to bind to the insulin receptors found within the body. Insulin-like growth factor 1 is one of the principal mediators of human growth hormone (GH). What this means is IGF-1 plays a crucial role in promoting cell growth, differentiation and has a distinct anabolic effect, especially in adults.
IGF-1 LR3 And Cell Proliferation
Since IGF-1 is a part of a wide growth factor network, it plays a part in cellular proliferation and differentiation, especially in muscles and connective tissue. It also delays cell apoptosis, preventing premature cell death.
The difference between IGF-1 and IGF-1 LR3 variant is, as we said before, LR3’s longer half life; it remains in the bloodstream longer and expresses its action over a longer period.
In vitro medical studies showed that this peptide leads to the increase of muscle cells, not by hypertrophy but by actual cell division. This “side effect” proved of interest in the bodybuilding world where individuals would use (abuse) it to improve their results and get results faster. It came to a point where this peptide was declared a doping agent and banned in different high level sports competitions.
Of course, this is not something we approve of, as we are only selling peptides for laboratory testing.
IGF-1 As A Medical Disease Marker
We’re used to talking about IGF-1 and its LR3 counterpart, in the context of medical therapy and treatment, but its levels might point to a medical problem – deficiency within the body. There are many different reasons for IGF-1 deficiency, but it’s suspected that the main one is GH and growth hormone receptor defects. This is especially common in children where primary IGF deficiency is detected and treated.
IGF-1 LR3’s Effects On Myostatin Regulation
Myostatin is a protein found in muscle tissue of the skeletal system, mainly responsible for hypertrophy and cell division and differentiation. We’re not talking about muscle building here, but normal bodily muscle development during the growth period.
Deficiencies with this hormone will lead to muscle dystrophies, where affected individuals experience muscle loss, decreased quality of life as well as life expectancy. This is why researchers are so hung up on developing a IGF-1 LR3 treatment protocol that would help regulate myostatin protein, prevent muscle loss and improve the quality of life of the patients.
Sadly, we are still in the early stages of testing and research – most commonly used models are animal ones, and we have yet to see the effects of this therapy in humans.
IGF-1 LR3 Effects On Cell Aging
In addition to cell proliferation, differentiation and wound healing, some animal studies showed IGF-1 LR3 affects cellular longevity (by protecting them and prolonging apoptosis).
Besides general wellbeing, this therapy can be aimed at slowing down the progression of deteriorating diseases such as dementia or muscle dystrophy. These results have been reported in mice studies, but we should move into human testing soon.
References:
Bailes J, Soloviev M. Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports. Biomolecules. 2021 Feb 4;11(2):217. doi: 10.3390/biom11020217. PMID: 33557137; PMCID: PMC7913862.
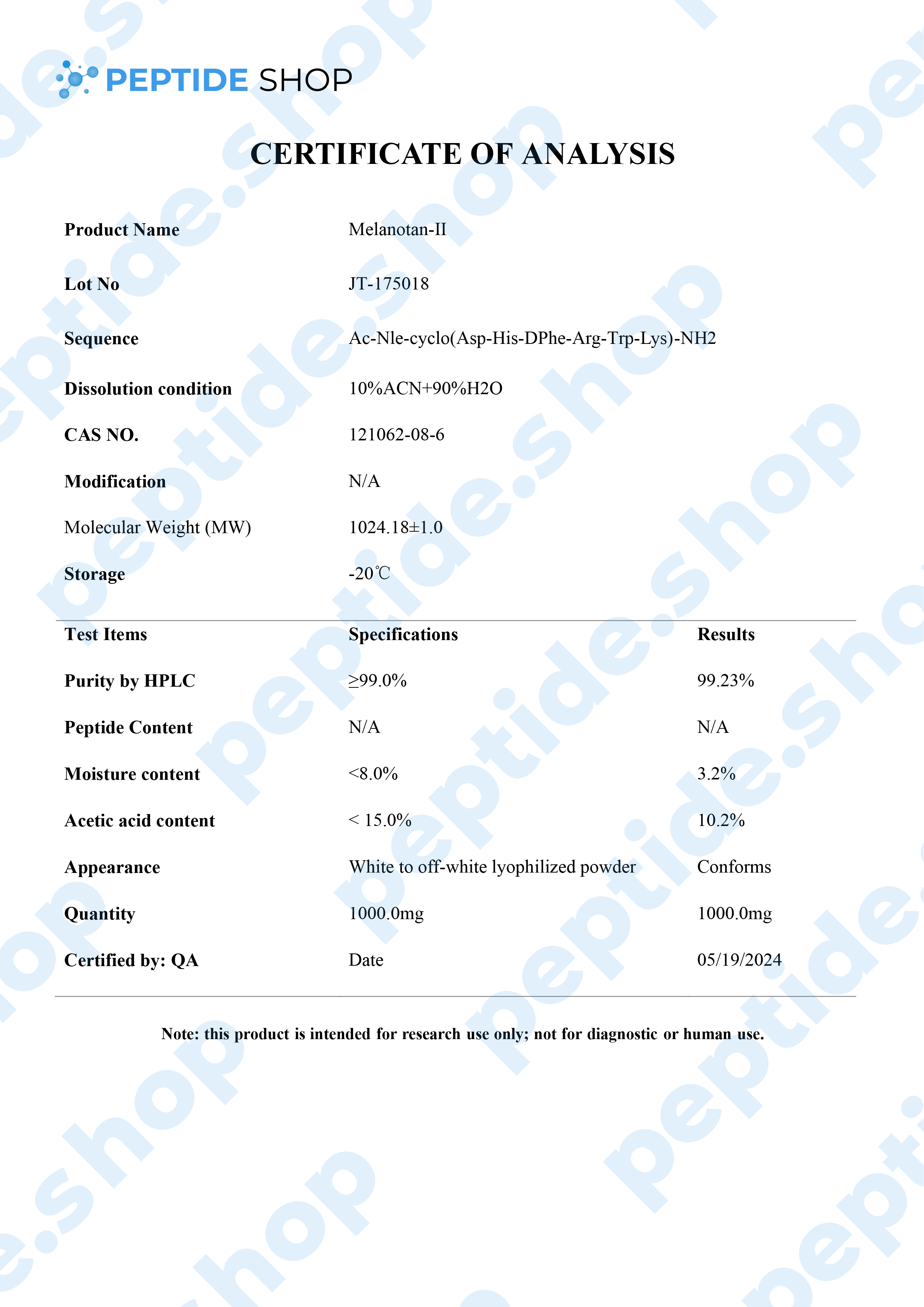
Melanotan 2 is a synthetic version of human alpha-melanocyte stimulating hormone and acts as a non selective melanocortin-receptor agonist. It was mainly developed as a sunless tanning option, but some clinical studies showed it also causes spontaneous penile erections, as well as general sexual stimulation.
Some of the other “side effects” researchers noticed were reduction in compulsive behavior, reduced glucagon production, hunger suppression and even some limited addiction control.
Melanotan 2 Effects On Autism
There is some additional recent clinical research suggesting there might be a link between Mealnotan 2 and autism. Of course, melanotan treatment cannot reverse autism, sadly, but it may act on some aspects of this disorder and make it more manageable.
In mouse model studies researchers recorded that MT2 stimulated oxytocin release, which had the potential to reduce (in some cases counteract) common ASD behaviors – behaviors that include:
- Hand clapping
- Bouncing on toes
- Body rocking
- Holding body parts in unusual positions
- Repeating vocalizations etc.
Semax is an interesting nootropic that can capture the attention of researchers, health professionals, and enthusiasts alike. It was originally developed in Russia back in the 1980s and 1990s. Nowadays, Semax is believed to enhance a range of cognitive and neuroprotective properties.
Many know this peptide by its trade name, such as Semaxum, and it has been extensively researched by institutions such as the Institute of Molecular Genetics of the Russian Academy of Science.
What is Semax?
Semax is a peptide best known for its neurogenic, neuroprotective, and nootropic properties. It was developed based on the molecular structure of ACTH or adrenocorticotropic hormone. Preliminary studies in small-scale human trials, animals, and cells indicate potential benefits of Semax use.
In the U.S. and multiple countries around the world, this component is the basis for a number of medicines that are used in clinical practices for the treatment of optic nerve atrophy, dys-circulatory encephalopathy, and ischemic brain stroke.
Potential benefits
Some of the benefits Semax may include:
- Increased short-term memory and attention
- Better non-proliferative diabetic neuropathy
- Can assist during recovery from hypoxia/stroke
- Can help with glaucoma optic neuropathy
- May act as an analgesic
- It may help in treating ADHD
- It may help protect the brain from various types of damage and stress
How Does Semax Work?
Semax increases BDNF or brain-derived neurotrophic factor. BDNF is one of the most active neurotrophins, which help control and stimulate neurogenesis, creating new neurons in the brain. This component helps support neuroplasticity, allowing nerve cells in the brain to adapt to new situations and recover from injury.
Additionally, BDNF encourages the survival of existing neurons and supports the growth, creation, and regeneration of new synapses and neurons.
This nootropic also helps minimize the breakdown of enkephalins. Enkephalins are mostly involved in reducing inflammation, decreasing pain, boosting immune cell activity, and preventing cancer cell growth.
They also play a role in pain, emotional behavior, learning, and memory. Balanced enkephalins are necessary to maintain normal brain function. Therefore, researchers are led to believe that Semax may potentially relieve pain. However, more research is needed to confirm the preliminary findings.
References:
Medvedeva EV, Dmitrieva VG, Povarova OV, Limborska SA, Skvortsova VI, Myasoedov NF, Dergunova LV. The peptide semax affects the expression of genes related to the immune and vascular systems in rat brain focal ischemia: genome-wide transcriptional analysis. BMC Genomics. 2014 Mar 24;15:228. doi: 10.1186/1471-2164-15-228. PMID: 24661604; PMCID: PMC3987924.
https://www.sciencedirect.com/science/article/abs/pii/S0306987706005391
Gusev, E. & Martinov, Michail & Kostenko, E. & Petrova, Lyudmila & Bobyreva, S.. (2018). The efficacy of semax in the tretament of patients at different stages of ischemic stroke. Zhurnal nevrologii i psikhiatrii im. S.S. Korsakova. 118. 61. 10.17116/jnevro20181183261-68.
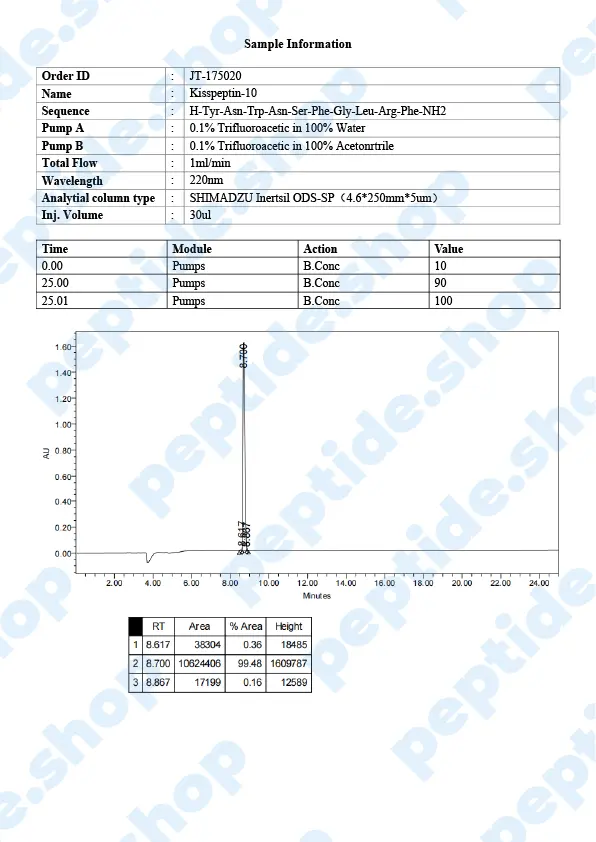
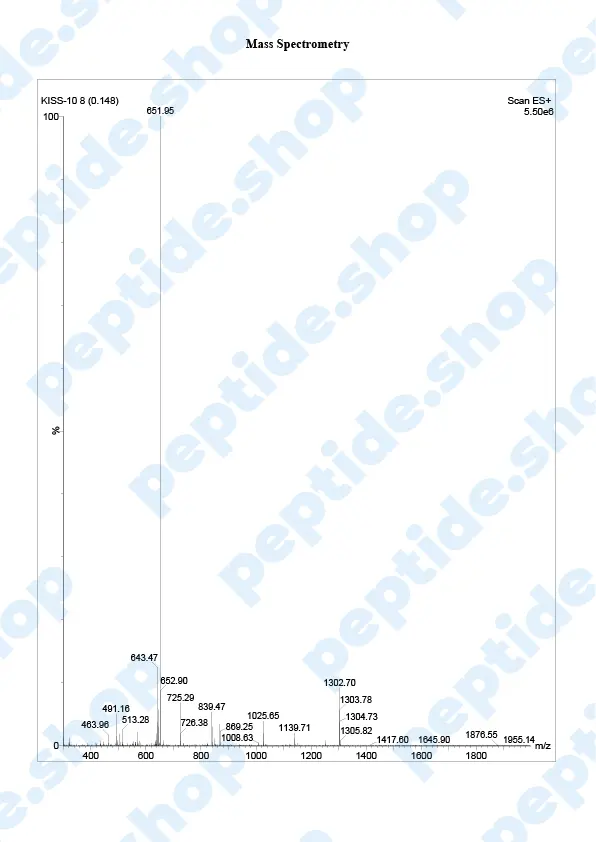
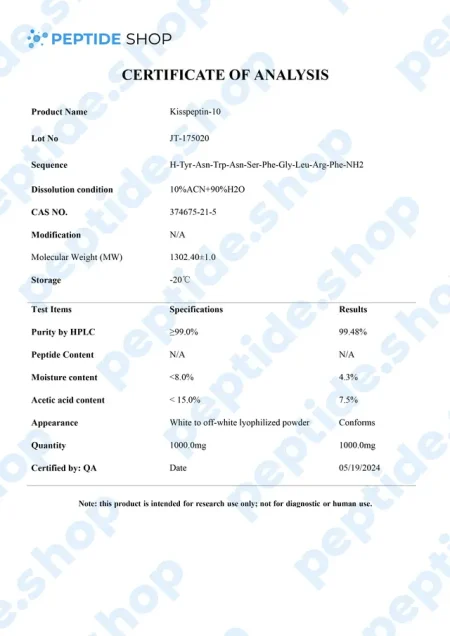
Metastin or Kisspeptin is believed to have the ability to prevent the spread of cancer or metastasis. This peptide is produced by the hypothalamus, which stimulates the release of GnRH or gonadotropin-releasing hormone, which then causes follicle-stimulating hormone and luteinizing hormone to be released from the purity gland.
Kisspeptin is used in testosterone replacement therapy instead of hCG to increase testosterone levels. That’s why it has emerged as a crucial regulator of the mammalian reproductive axis.
This peptide was first discovered in 1996 when it managed to inhibit melanoma cell lines. It belongs to a family of peptides that are derived from the KISS1/kiss1 gene structure, forming from prepro-kisspeptin, which has differential proteolysis for a common precursor.
Kisspeptin is classified as an RF or neuroactive peptide with a specific Arg-Phe-NH2 motif.
Benefits:
Kisspeptin binds to receptors in the pituitary gland, triggering a response that prompts the gland to release neurotransmitters, signaling the release of LH and FSH.
Based on numerous animal studies, scientists have managed to uncover the following benefits:
- Boosts the production of testosterone naturally
- Regulates fertility
- Increases sexual drive
- Improves immune response
- Boosts brain function
- Promotes weight loss
How does Kisspeptin work?
This peptide stimulates the release of GnRH or gonadotropin-releasing hormone. It enters the purity gland through receptor sites, causing a gland to release neurotransmitters, which later signal the releases of FH and LH. These hormones play a crucial role in the production of oestradiol and testosterone.
Kisspeptin mimics the action of hCG and clomiphene, and according to some animal studies, it can affect fertility in female and male animals.
Distribution of Kisspeptin
Kisspeptin was first found in the placenta, and later, it has been observed in the small intestine, pancreas, ovaries, and testis in mammals. The primary expression of Kisspeptin and its receptors have been shown in two big neuronal populations within the hypothalamus of rodents, the anteroventral periventricular nucleus and the arcuate nucleus.
Growth hormone replacement therapy has long been accepted as one of the most effective ways of treating GH deficiency and related conditions. This therapy uses recombinant human growth hormone (rhGH) and, despite its longstanding usage record, it has been surrounded by controversy. Even though, in the short term, this therapy is very effective, when it comes to long term effects, issues start to arise:
- Long term improper dosage can lead to some severe side effects
- This therapy may affect normal physiology after prolonged application
- Some federal regulations might interfere with the therapy
- There’s even some concerns that this therapy may awaken latent cancers and cause metabolic disorders
There is one more major problem with dosage because the body can’t properly modulate tissue exposure to rhGH. Meaning, the practitioner uses their “best guess” to access the appropriate dosage.
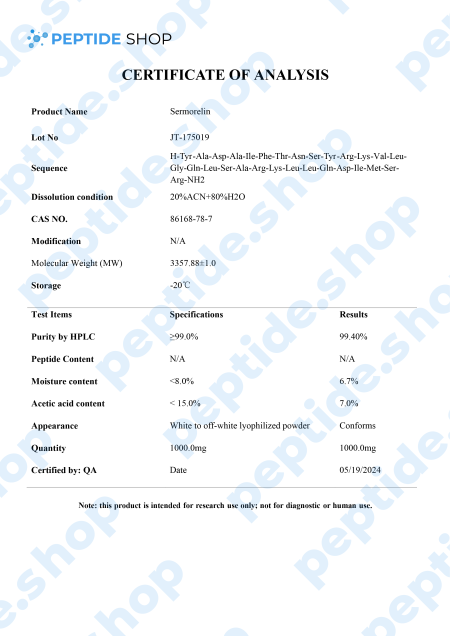
This is why researchers are looking for alternative ways to stimulate GH production by targeting GH secretagogues and promoting pituitary gland health. One such molecule is sermorelin, a GHRH analog that impacts the hypothalamic-pituitary-pituitary-somatotropic axis.
It’s used for both treatment and diagnosis of GH deficiency as research studies showed sermorelin promoted change in GH levels similar to those observed with endogenous GHRH. It also stimulates FSH (follicle-stimulating hormone) and LH (luteinizing hormone), which implies a potential role in the treatment of hypogonadism.
Though we need larger, longitudinal studies, sermorelin proved as a promising GH and IGF-1 stimulator and diagnostic agent.
Reference:
Walker RF. Sermorelin: a better approach to management of adult-onset growth hormone insufficiency? Clin Interv Aging. 2006;1(4):307-8. doi: 10.2147/ciia.2006.1.4.307. PMID: 18046908; PMCID: PMC2699646.
Sinha DK, Balasubramanian A, Tatem AJ, Rivera-Mirabal J, Yu J, Kovac J, Pastuszak AW, Lipshultz LI. Beyond the androgen receptor: the role of growth hormone secretagogues in the modern management of body composition in hypogonadal males. Transl Androl Urol. 2020 Mar;9(Suppl 2):S149-S159. doi: 10.21037/tau.2019.11.30. PMID: 32257855; PMCID: PMC7108996.
Obesity’s become a global pandemic, currently affecting one third of the entire population, and this is why we can look at it as a chronic disease that requires appropriate treatment.
When talking about obesity, amylin hormone is of great importance as it gets secreted along with insulin and acts as food intake inhibitor, delaying gastric emptying and suppresses post-prandial glucagon responses to meals.
For this reason, there is a tendency to include amylin management in newly developed medication.
And one such compound is cagrilintide – lipidated long-acting amylin analogue.
Though it still hadn’t been thoroughly tested on humans, numerous rat studies showed incredible potential. One such in vitro study involving rats set out to compare cagrilintide’s effectiveness against pramlintide (an FDA-approved diabetes 1/2 medication).
The study showed that pramlintide reduced food intake by 25% in the period of 0-24 hours (it did not cause reduced food intake after 24 hours). But the dosage was substantial – 1000 nmol/kg.
On the other hand, cagrilintide was able to reduce food intake by approximately 50% with a minimal dosage of only 3 nmol/kg. More importantly, this food intake reduction spanned across 60 hours from the moment it was injected.
These results clearly show the potency of cagrilintide in weight loss and diabetes management medication, especially because it acts over such a long time period (allowing subcutaneous injections to be applied once a week).
Reference:
Dehestani B, Stratford NR, le Roux CW. Amylin as a Future Obesity Treatment. J Obes Metab Syndr. 2021 Dec 30;30(4):320-325. doi: 10.7570/jomes21071. PMID: 34929674; PMCID: PMC8735818.
Numerous studies have shown that this peptide may represent numerous opportunities for competent scientists to investigate, especially cartilage regeneration and fatty tissue mitigation.
What is Fragment 176-191 peptide?
This synthetic amino acid sequence mimics the hGH sequence, starting from position 177 to position 191.
AOD 9604, or “lipolytic extract,” is another name for Fragment 176-191. Many researchers argue that Fragment 177-191 may be an important research compound in the context of obesity. They believe it can potentially burn fat in animal models in laboratory settings.
Despite positive results, Fragment 176-191 hasn’t been used for human research purposes and doesn’t have official medical approvals.
Growth hormone
According to research, it appears that hGH Fragment 176-191 may not produce any side effects often related to hGH supplementation. Several adverse effects, such as, impaired glucose tolerance, insulin resistance, and increases in IGF-a levels, have cast doubt on hGH’s use as an obesity treatment. However, when it comes to hGH Fragment 176-191, it doesn’t seem to be impacting blood IGF-1 levels or negatively affecting carbohydrate metabolism.
A trial conducted on Zucker rats in 2000 showed no detrimental effects on insulin sensitivity. Also, an investigation on obese mice done in 2001 did not seem to affect insulin secretion.
Properties
Studies conducted on animals suggest that this peptide can exhibit significant theoretical properties, which will be outlined below:
- The main potential of this Fragment is its lipolytic properties or, in other words, fat-reducing potential. While growth hormones promote development in infancy, they have also been speculated to have an important purpose in maturity.
- Lipoprotein lipase inhibitors may be activated in fat cells,
- By stimulating lipolysis in adipocytes may lead to a decrease in fat cell bulk,
- It might lead to loss of fat from the host body,
Weight
A 14-day trial researching overweight mice came to a conclusion that Fragment 176-191 may have increased skeletal muscle thermogenesis and boosted fat burning.
The study results indicated that this peptide may have enhanced beta (3)-AR RNA (ADRB3) levels, which caused rapid weight reduction in overweight animals but didn’t have much effect on lean mice.
Cartilage
Based on other animal research, it appears that hGH Fragment 176-191 may boost the effects of hyaluronic acid (HA). When Fragment 176-191 was combined with hyaluronic acid, it might boost cartilage formation in white rabbits which have issues with osteoporosis.
References:
Habibullah MM, Mohan S, Syed NK, Makeen HA, Jamal QMS, Alothaid H, Bantun F, Alhazmi A, Hakamy A, Kaabi YA, Samlan G, Lohani M, Thangavel N, Al-Kasim MA. Human Growth Hormone Fragment 176-191 Peptide Enhances the Toxicity of Doxorubicin-Loaded Chitosan Nanoparticles Against MCF-7 Breast Cancer Cells. Drug Des Devel Ther. 2022 Jun 27;16:1963-1974. doi: 10.2147/DDDT.S367586. PMID: 35783198; PMCID: PMC9249349.
https://www.sciencedirect.com/science/article/abs/pii/0304416582900332
Habibullah, Mahmoud & Mohan, Syam & Syed, Nabeel & Makeen, Hafiz & Jamal, Qazi & Alothaid, Hani & Bantun, Farkad & Hakamy, Ali & Kaabi, Yahia & Samlan, Ghalia & Lohani, Mohtashim & Thangavel, Neelaveni & Al-Kasim, Mohamed. (2022). Human Growth Hormone Fragment 176–191 Peptide Enhances the Toxicity of Doxorubicin-Loaded Chitosan Nanoparticles Against MCF-7 Breast Cancer Cells. Drug Design, Development and Therapy. 16. 1963. 10.2147/DDDT.S367586.
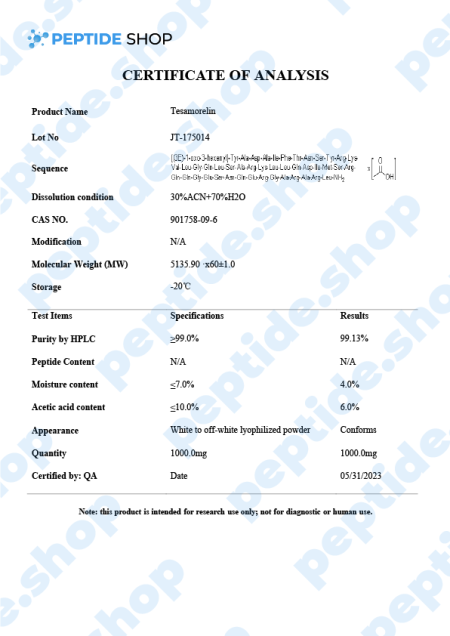
Tesamorelin is a 44 amino acid long, synthetic growth hormone releasing hormone (GHRH) analogue. It was primarily developed and used to treat visceral fat buildup in HIV positive patients suffering from lipodystrophy (a condition characterized by abnormal fat distribution).
Tesamorelin peptide activates GHRH receptors in the pituitary gland, resulting in growth hormone synthesis and release. This GH release further stimulates the production of Ilike growth factor-1 (IGF-1), which is naturally low in obese and diabetic patients.
The good thing about tesamorelin is that it was approved in the US back in 2010 for the treatment of abdominal fat in HIV positive patients as a part of the antiviral therapy-related lipodystrophy. It has also been evaluated as a potential therapy of insulin resistance, nonalcoholic fatty liver and, of course, obesity. These clinical studies are still ongoing and we’ll need more information to confirm its effectiveness.
The usual tesamorelin dosage for patients is 2mg given in the form of a subcutaneous injection, once a week. As for the side effects, patients did not report that many, but from the ones we have documented, most common are:
- Application site irritation
- Itching
- Peripheral edema
- Mild nausea
- Redness
More importantly, tirzepatide therapy was not associated with hepatotoxicity and is very unlikely to cause any clinically apparent liver injuries.
Reference:
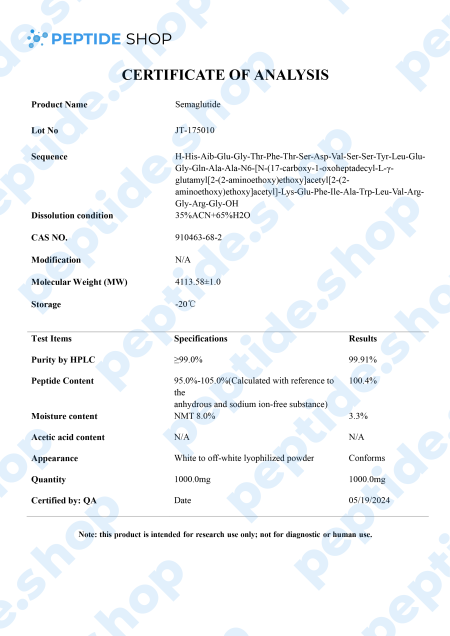
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist.It is one of the two primary hormones that may enhance incretin activity in rodent models, along with GIP.
GLP-1 is one of the major incretin (gut peptide secreted after nutrient intake) hormones in humans. It plays a pivotal role in many different mechanisms within the body:
- Insulin secretion
- Inhibits glucagon release (glucagon is secreted as a response in blood sugar drop)
- Suppresses hepatic gluconeogenesis (glycogen is the primary carbohydrate stored in the liver)
- Delays gastric emptying
- Reduces appetite and energy intake
When talking about obese diabetic 2 type patients, GLP-1 assumes a special status in their treatment as it lowers HbA1c levels along with body weight, but without the risk of hypoglycemia.
However, the biggest problem with GLP-1 is its short half-live (of only 1 to 2 minutes) and this is why researchers turner to various other GLP-1 receptor agonists, such as liraglutide, dulaglutide and, of course, semaglutide. Another thing researchers hope to achieve by turning to GLP-1 agonists, such as semaglutide, is to develop an effective diabetes management drug which needs less frequent dosing.
Murine models suggested that semaglutide’s GLP-1 receptor activation affects insulin secretion, blood sugar homeostasis and beta cell protection. Further, in vitro studies showed its potential to also affect glucagon secretion. This insulin glucagon mechanics is what’s used to balance our pancreatic function and regulate metabolism.
References:
- Mahapatra MK, Karuppasamy M, Sahoo BM. Semaglutide, a glucagon like peptide-1 receptor agonist with cardiovascular benefits for management of type 2 diabetes. Rev Endocr Metab Disord. 2022 Jun;23(3):521-539. doi: 10.1007/s11154-021-09699-1. Epub 2022 Jan 7. PMID: 34993760; PMCID: PMC8736331.
- Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012 Dec;8(12):728-42. doi: 10.1038/nrendo.2012.140. Epub 2012 Sep 4. PMID: 22945360.
500 in stock
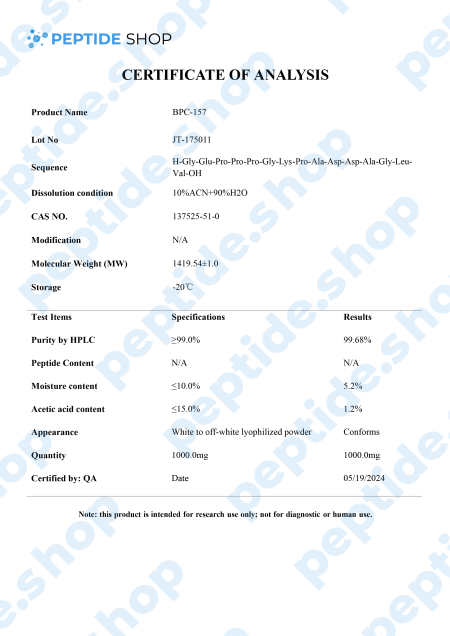
BPC-157 is a native gastric pentadecapeptide (composed of 15 amino acids), derivative of body protection compound (BPC). Being a gastric peptide allows for good oral availability and remaining stable for more than 24 hours in human gastric juice. Furthermore, there is no need for a carrier which makes this peptide unique, as compared to others that depend on it.
Clinical studies showed that BPC-157 is highly effective in both treatment and prophylaxis of various gastrointestinal lesions. This effectiveness spans to cover both acute and chronic gastric conditions, intravenous, ingastric, and even as a topical agent for deep skin burns and lesions.
Surprisingly, BPC-157 has also shown a beneficial effect on:
- Brain lesions – in one of the most recent studies researchers concluded that BPC-157 expressed a direct therapeutic effect in rat test subjects following a stroke. This peptide not only delayed neural damage, but also promoted full functional recovery.
- Behavioral disorders – medical research showed that BPC-157 counteracted catalepsy (a state of a trance or a seizure) in rat models. This research indicated a connection between BPC-157 and dopamine as well as glutamate and nitric oxide system (vital in schizophrenia therapy).
- Spinal cord injuries – A particularly interesting study showed that BPC-157 administration to rats with spinal cord injuries resulted in improvements mere 10 minutes after the initial dosage. On the contrary, untreated rats did not fully recover days, weeks, months and, in some rare cases, years following the injury.
In conclusion, we saw BPC-157 application results in a myriad of beneficial effects in various different systems within the body. Of course, many of these studies were performed on animal test subjects and we’re going to need additional ones to clarify its effect in humans and the full extent of its therapeutic potential.
References:
Vukojevic J, Milavić M, Perović D, Ilić S, Čilić AZ, Đuran N, Štrbe S, Zoričić Z, Filipčić I, Brečić P, Seiverth S, Sikirić P. Pentadecapeptide BPC 157 and the central nervous system. Neural Regen Res. 2022 Mar;17(3):482-487. doi: 10.4103/1673-5374.320969. PMID: 34380875; PMCID: PMC8504390.
Sikiric P, Hahm KB, Blagaic AB, Tvrdeic A, Pavlov KH, Petrovic A, Kokot A, Gojkovic S, Krezic I, Drmic D, Rucman R, Seiwerth S. Stable Gastric Pentadecapeptide BPC 157, Robert’s Stomach Cytoprotection/Adaptive Cytoprotection/Organoprotection, and Selye’s Stress Coping Response: Progress, Achievements, and the Future. Gut Liver. 2020 Mar 15;14(2):153-167. doi: 10.5009/gnl18490. PMID: 31158953; PMCID: PMC7096228.
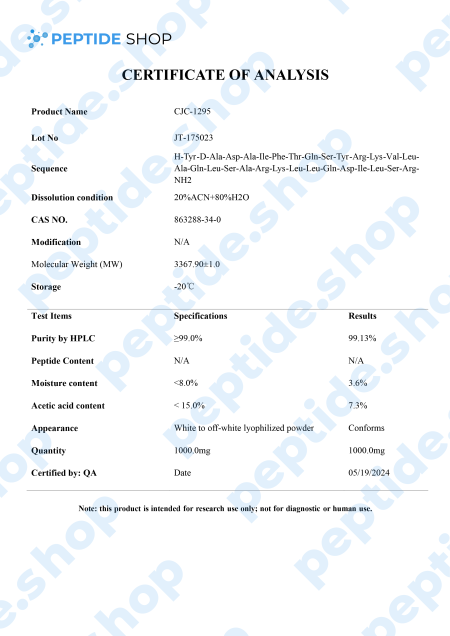
CJC-1295 is a synthetic analogue of growth hormone-releasing hormone (GHRH) and growth hormone secretagogue (GHS) developed by ConjuChem Biotechnologies.
GHRH is a 44-amino acid long peptide which our hypothalamus synthesizes and in the pituitary gland, it binds to the growth hormone (GH) receptors, resulting in the release, regulation and pulsatile secretion of GH.
We already talked about GH therapy as being FDA approved in conditions such as GH deficiency, Turner syndrome, Prader-Willi syndrome, idiopathic short stature etc. Recombinant human GH treatment is generally performed as one daily, subcutaneous injection which elevates the levels of GH serum in the blood.
One of the major problems with this approach to treatment is that the efficacy of GH therapy is hard to determine due to the lack of biological serum biomarkers. Currently, most facilities are using levels of IGF-1 and IGFBP-3 to monitor the efficacy of this therapy but these levels may vary wildly (due to growth velocity, glucose tolerance, insulin levels etc.).
Furthermore, GH abuse extended across multiple sports disciplines, making it even harder to suppress and put this problem under control.
Researchers hope to solve this problem by employing new biomarkers of GH action and secretion. One such way is to employ newly developed molecules, such as CJC-1295, shown to increase both GH and IGF-1 levels in the blood, without affecting the pulsatility of GH secretion. CJC-1295 also has a prolonged half-life of 8 to 10 days, due to its ability to bind to the endogenous serum albumin.
So, what scientists are hoping to achieve with CJC-1295 is both a safe way of promoting GH secretion as well as making it measurable in a laboratory setting. There were numerous studies tackling this issue, but we still need further testing to confirm these preliminary findings.
Reference:
Sackmann-Sala L, Ding J, Frohman LA, Kopchick JJ. Activation of the GH/IGF-1 axis by CJC-1295, a long-acting GHRH analog, results in serum protein profile changes in normal adult subjects. Growth Horm IGF Res. 2009 Dec;19(6):471-7. doi: 10.1016/j.ghir.2009.03.001. Epub 2009 Apr 21. PMID: 19386527; PMCID: PMC2787983.
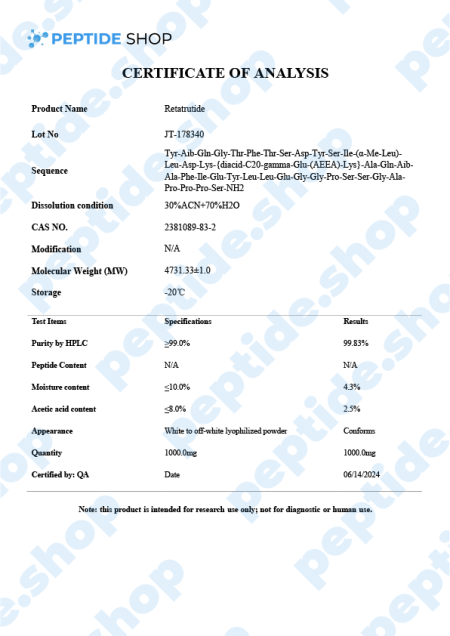
Retatrutide is one of the latest incretin mimetic drugs being tested for their weight reduction properties. Researchers use incretin mimetic agents as they work to mimic incretin hormones (gut peptides that get secreted after we eat) to allow for a better insulin secretion and hyperglycaemic control.
Even though there are numerous weight reduction agents being prescribed and used, FDA-recognized “treatment” for nonsyndromic obesity are:
- Orlistat
- Phentermine-topiramate
- Naltrexone‐bupropion
- Liraglutide and
- Semaglutide
With the exception of glucagon-like peptide 1 receptor agonists (such as semaglutide), most weight reduction agents come with a specific set of side effects such as nausea, gastrointestinal distress, abdominal pain, vomiting, diarrhea, or a general weight decrease that does not cross the 15% mark. Some of these medications even come with a warning against using them if a patient is suffering from a kidney, heart, thyroid or any related problems.
This is why scientists are turning more and more to retatrutide, because this incretin mimetic decreases appetite, promotes the feeling of fullness and slows down the process of gastric emptying, but does all that while presenting with minimal side effects and increased weight loss effectiveness, compared to liraglutide or semaglutide.
Retatrutide Mechanism of Action
Retatrutide exerts a powerful glucagon-receptor agonistic effect. When we compare it to human glucagon and glucagon-like peptide 1 (GLP-1), it exhibits:
- Reduced potency on the GCGR (human glucagon receptor) and GLP-1R (glucagon-like peptide-1 receptor)
- Enhanced potency at the human GIPR (gastric inhibitory polypeptide receptor)
When talking about in vitro experiments, retatrutide demonstrated similar efficacy in evoking glucose production within hepatocytes and inducing lipolysis. Also, retatrutide showed a favorable half-life of 6 days, enabling it a weekly dosage regimen (should the drug get FDA approved).
Another double-blind study conducted in the US on obese patients showed promising results. 338 adults in total were involved, split into 6 cohorts (depending on the weekly dosage), up against a placebo group. Retatritude group experienced the following results:
- 1mg group – after 24 weeks this group experienced weight loss of 7.2%, and after 48 weeks a total loss of 8.7%
- 4mg group (commencing with 2mg) – after 24 weeks this group experienced a weight loss of 11.8%, and after 48 weeks a total loss of 16.3%
- 4mg group (commencing with 4mg) – after 24 weeks this group experienced a weight loss of 13.9%, and after 48 weeks a total loss of 17.8%
- 8mg group (commencing with 2mg) – after 24 weeks this group experienced a weight loss of 16.7%, and after 48 weeks a total loss of 21.7%
- 8mg group (commencing with 4mg) – after 24 weeks this group experienced a weight loss of 17.9%, and after 48 weeks a total loss of 23.9%
- 12mg group (commencing with 2mg) – after 24 weeks this group experienced a weight loss of 17.5%, and after 48 weeks a total loss of 24.2%
In contrast, the placebo group only experienced a 1.6% weight loss after 24 weeks and 2.1% after 48. Findings also showed that adverse effects related to retratitude treatment (such as nausea, vomiting, diarrhea and constipation), were mild and depended on the dosage.
In conclusion, this peptide showed promising results in addressing obesity in both in vitro and human test subject studies. However, test samples were relatively small, and we still need more research and clinical trials to show the effectiveness of retratutide as an effective, long-term way of managing weight.
Reference:
Naeem M, Imran L, Banatwala UESS. Unleashing the power of retatrutide: A possible triumph over obesity and overweight: A correspondence. Health Sci Rep. 2024 Feb 5;7(2):e1864. doi: 10.1002/hsr2.1864. PMID: 38323122; PMCID: PMC10844714.

 Anti Aging
Anti Aging Hair Growth
Hair Growth Muscle Growth
Muscle Growth Peptide Blends
Peptide Blends Peptide Supplies
Peptide Supplies Peptides
Peptides Skin
Skin Testosterone
Testosterone Weight Loss
Weight Loss











Reviews
There are no reviews yet.