Oxytocin 10mg
Oxytocin, also known as the love hormone, helps us bond with our loved ones and is often released through touch, exercise, and music.
What is Oxytocin?
This natural hormone manages crucial aspects of male and female reproductive systems, including lactation, labor, delivery, and certain aspects of human behavior. The hypothalamus produces oxytocin, but the posterior pituitary gland releases and stores this hormone into the bloodstream.
Hormones are chemicals that manage various functions in the human body by carrying messages through blood to tissues, muscles, and organs. These signals instruct your body what to do and when to do it.
The hypothalamus is part of the brain that controls functions such as heart rate, digestion, body temperature, and blood pressure. On the other hand, the pituitary gland is a small, pea-sized endocrine gland placed at the base of the brain, below the hypothalamus.
Synthetic Forms of Oxytocin
A synthetic form of oxytocin is used in hospital settings, particularly when doctors need to induce labor in childbirth if it hasn’t begun naturally or to help with contractions. Healthcare providers may also use this hormone to speed up the delivery of the placenta, which is the third stage of labor, and minimize the risk of heavy bleeding.
Function of Oxytocin
As previously mentioned, the two main functions of oxytocin are to encourage uterine contractions in childbirth and labor and to boost contractions of breast tissue to help with lactation after childbirth.
However, apart from these, oxytocin has other important roles, such as:
- Placenta-infant bonding
- Romantic attachment
- Trust
- Recognition
- Sexual arousal
The effects of oxytocin on the human brain are complex, and scientists are currently researching the role of oxytocin in various conditions.
- PTSD
- Depression
- Autism spectrum disorder
- Anxiety
- Anorexia
- Addiction
Florea T, Palimariciuc M, Cristofor AC, Dobrin I, Chiriță R, Bîrsan M, Dobrin RP, Pădurariu M. Oxytocin: Narrative Expert Review of Current Perspectives on the Relationship with Other Neurotransmitters and the Impact on the Main Psychiatric Disorders. Medicina (Kaunas). 2022 Jul 11;58(7):923. doi: 10.3390/medicina58070923. PMID: 35888641; PMCID: PMC9318841.
Kendrick KM, Guastella AJ, Becker B. Overview of Human Oxytocin Research. Curr Top Behav Neurosci. 2018;35:321-348. doi: 10.1007/7854_2017_19. PMID: 28864976.
https://www.sciencedirect.com/science/article/pii/S0306453013002369
$50.00
| Quantity: | 10mg |
|---|---|
| Unit: | 1 vial |
| Contents: | Oxytocin |
| Form/Appearance: | Lyophilized/Powder |
| Peptide Purity: | 99% |
| Sequence: | Cys(1)-Tyr-Ile-Gln-Asn-Cys(1)-Pro-Leu-Gly |
| Molecular Mass: | 1007.193 g/mol |
| Solubility: | Sterile / Bacteriostatic water |
| Synonyms: | Pitocin, Endopituitrina, Ocytocin |
Based on 0 reviews
|
|
|
0% |
|
|
|
0% |
|
|
|
0% |
|
|
|
0% |
|
|
|
0% |
FAQs
Related Products
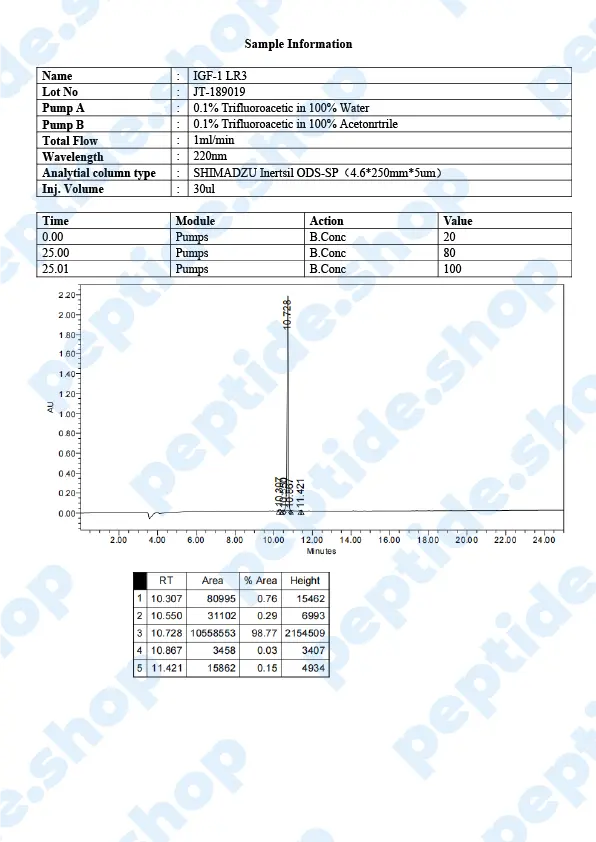
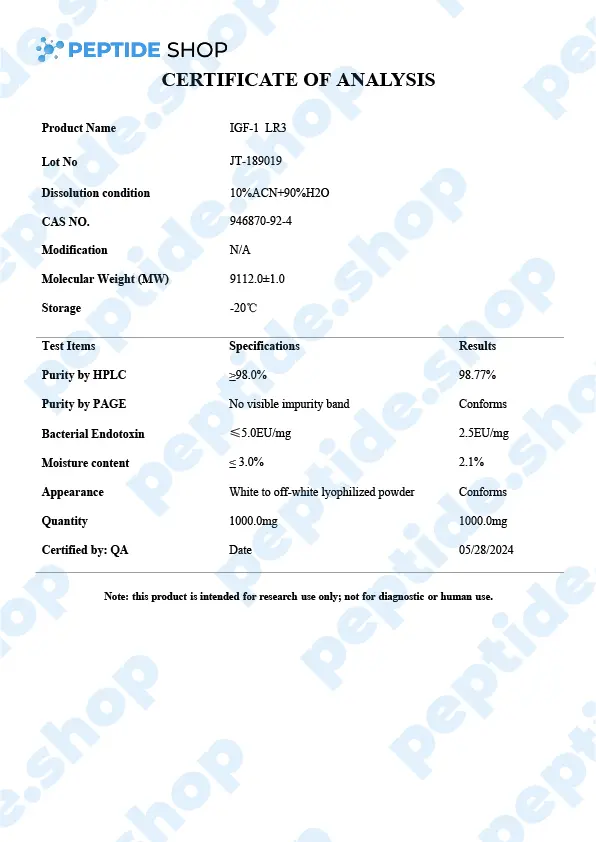
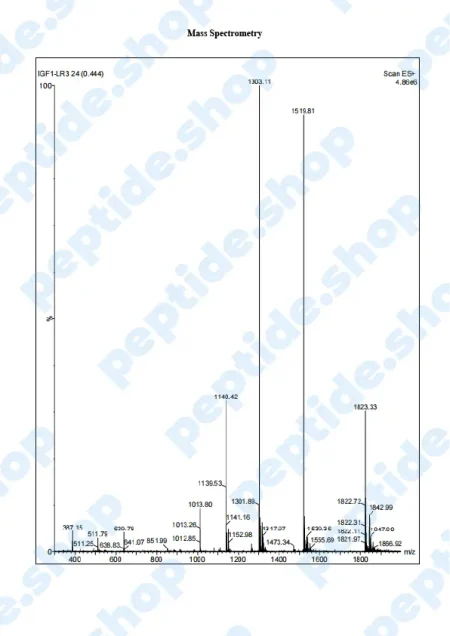
PeptideShop.com is selling insulin-like growth factor 1 (IGF-1) LR3 in vials of 3mg, in a lyophilized state, and it requires dissolution. Your choice of diluent will depend on the nature of your lab testing, but the most common ones are certainly sterile distilled water, or a sterile dilute acetic acid.
IGF-1 LR3 is based on IGF which has been chemically altered to have a prolonged half-life. This allows it to be more effective and increases the duration of its action.
IGF-1 is a 70 amino acid long peptide chain, similar in structure to insulin, which is able to bind to the insulin receptors found within the body. Insulin-like growth factor 1 is one of the principal mediators of human growth hormone (GH). What this means is IGF-1 plays a crucial role in promoting cell growth, differentiation and has a distinct anabolic effect, especially in adults.
IGF-1 LR3 And Cell Proliferation
Since IGF-1 is a part of a wide growth factor network, it plays a part in cellular proliferation and differentiation, especially in muscles and connective tissue. It also delays cell apoptosis, preventing premature cell death.
The difference between IGF-1 and IGF-1 LR3 variant is, as we said before, LR3’s longer half life; it remains in the bloodstream longer and expresses its action over a longer period.
In vitro medical studies showed that this peptide leads to the increase of muscle cells, not by hypertrophy but by actual cell division. This “side effect” proved of interest in the bodybuilding world where individuals would use (abuse) it to improve their results and get results faster. It came to a point where this peptide was declared a doping agent and banned in different high level sports competitions.
Of course, this is not something we approve of, as we are only selling peptides for laboratory testing.
IGF-1 As A Medical Disease Marker
We’re used to talking about IGF-1 and its LR3 counterpart, in the context of medical therapy and treatment, but its levels might point to a medical problem – deficiency within the body. There are many different reasons for IGF-1 deficiency, but it’s suspected that the main one is GH and growth hormone receptor defects. This is especially common in children where primary IGF deficiency is detected and treated.
IGF-1 LR3’s Effects On Myostatin Regulation
Myostatin is a protein found in muscle tissue of the skeletal system, mainly responsible for hypertrophy and cell division and differentiation. We’re not talking about muscle building here, but normal bodily muscle development during the growth period.
Deficiencies with this hormone will lead to muscle dystrophies, where affected individuals experience muscle loss, decreased quality of life as well as life expectancy. This is why researchers are so hung up on developing a IGF-1 LR3 treatment protocol that would help regulate myostatin protein, prevent muscle loss and improve the quality of life of the patients.
Sadly, we are still in the early stages of testing and research – most commonly used models are animal ones, and we have yet to see the effects of this therapy in humans.
IGF-1 LR3 Effects On Cell Aging
In addition to cell proliferation, differentiation and wound healing, some animal studies showed IGF-1 LR3 affects cellular longevity (by protecting them and prolonging apoptosis).
Besides general wellbeing, this therapy can be aimed at slowing down the progression of deteriorating diseases such as dementia or muscle dystrophy. These results have been reported in mice studies, but we should move into human testing soon.
References:
Bailes J, Soloviev M. Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports. Biomolecules. 2021 Feb 4;11(2):217. doi: 10.3390/biom11020217. PMID: 33557137; PMCID: PMC7913862.
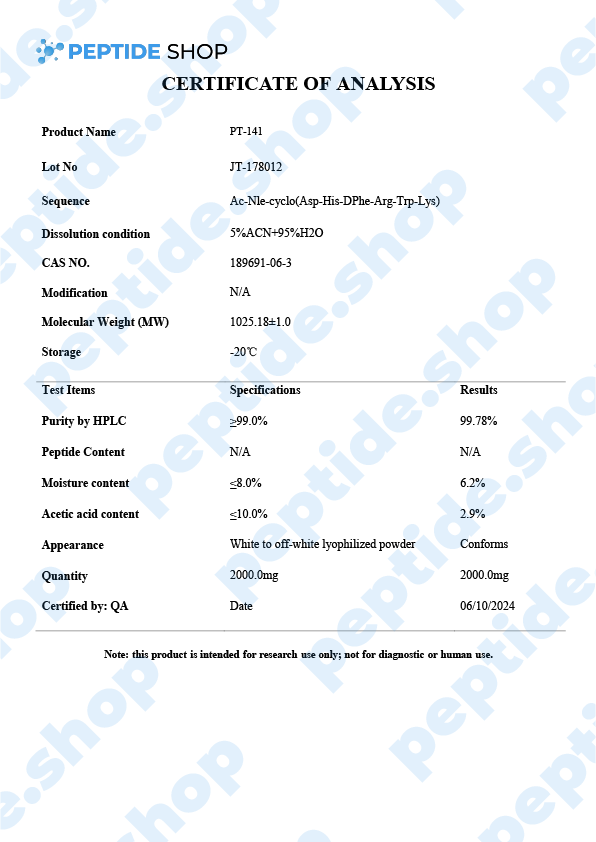
Also called Bremelanotide, PT-141 is a synthetic melanocortin receptor agonist that promotes dopamine release. Since it has such a high affinity for MC4R (Melanocortin 4 Receptor), in the presynaptic neurons of the hypothalamus, it has been approved as a treatment for HSDD (hypoactive sexual desire disorder) in premenopausal women. Under the brand name Vyleesi, Bremelanotide is the first and only FDA-approved way of HSDD treatment.
Sexual Dysfunctions
As we already said, PT-141 is an effective treatment for HSDD in premenopausal women but it’s important to note it should not be used as a treatment in women who already went through menopause or as a way of boosting sexual performance in men. These have not yet been fully tested.
We’ve seen the effectiveness of PT-141 (Bremelanotide) demonstrated in numerous studies. One such study followed female test subjects over the course of 52 weeks where the treated group received a 1.75mg dose right before anticipated sexual intercourse. All participants showed higher scores on general arousal, desire and orgasm, as compared to the placebo group. Also, no major side effects were linked to PT-141 application – the “most severe” ones were nausea, flushing and headache, which only occurred in around 10% of the participants.
Interestingly enough, scientists are still unsure about the Bremelanotide’s exact mechanism of action and how it leads to increased sexual desire in female patients. All we know is that it’s a potent alpha melanocyte-stimulating hormone, binding predominantly to the receptors MC4R and MC1R, and, to a certain extent, to MC1R-MC5R.
PT-141 And Erectile Dysfunction
At the beginning of the article we said that PT-141 (bremelanotide) should not be used to increase sexual desire in men… but this is not entirely true as there is some emerging evidence that this peptide does affect male sexual performance.
Currently, there are a number of effective ED treatment options available. One of the most fail-proof and common ones, incidentally the most invasive one, is an intracavernosal injection – where an injection is applied directly to corpus cavernosum (spongy tissue that runs through the shaft of the penis).
Naturally, researchers are on the lookout for a new, less invasive treatment option and are testing PT-141 as such. Though research is still in its early stages, results showed significant increases in both duration and the erection quality in both “regular” men, as well as those known to be taking viagra.
We still need more research to confirm all these findings and determine the safety and efficacy of using PT-14 as a reliable ED treatment option, but clinical studies suggest we are heading in this direction.
https://www.sciencedirect.com/topics/medicine-and-dentistry/bremelanotide
Growth hormone replacement therapy has long been accepted as one of the most effective ways of treating GH deficiency and related conditions. This therapy uses recombinant human growth hormone (rhGH) and, despite its longstanding usage record, it has been surrounded by controversy. Even though, in the short term, this therapy is very effective, when it comes to long term effects, issues start to arise:
- Long term improper dosage can lead to some severe side effects
- This therapy may affect normal physiology after prolonged application
- Some federal regulations might interfere with the therapy
- There’s even some concerns that this therapy may awaken latent cancers and cause metabolic disorders
There is one more major problem with dosage because the body can’t properly modulate tissue exposure to rhGH. Meaning, the practitioner uses their “best guess” to access the appropriate dosage.
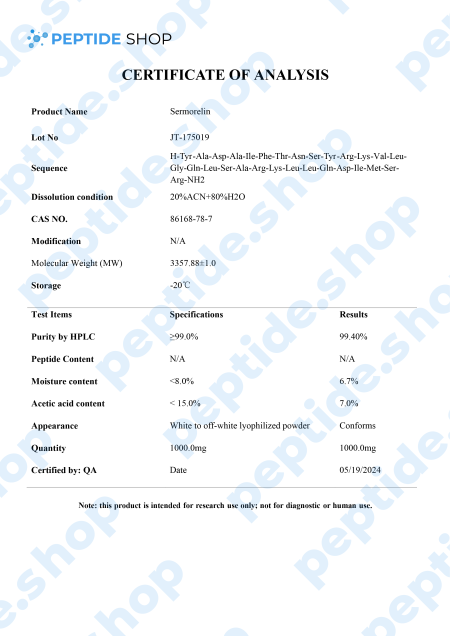
This is why researchers are looking for alternative ways to stimulate GH production by targeting GH secretagogues and promoting pituitary gland health. One such molecule is sermorelin, a GHRH analog that impacts the hypothalamic-pituitary-pituitary-somatotropic axis.
It’s used for both treatment and diagnosis of GH deficiency as research studies showed sermorelin promoted change in GH levels similar to those observed with endogenous GHRH. It also stimulates FSH (follicle-stimulating hormone) and LH (luteinizing hormone), which implies a potential role in the treatment of hypogonadism.
Though we need larger, longitudinal studies, sermorelin proved as a promising GH and IGF-1 stimulator and diagnostic agent.
Reference:
Walker RF. Sermorelin: a better approach to management of adult-onset growth hormone insufficiency? Clin Interv Aging. 2006;1(4):307-8. doi: 10.2147/ciia.2006.1.4.307. PMID: 18046908; PMCID: PMC2699646.
Sinha DK, Balasubramanian A, Tatem AJ, Rivera-Mirabal J, Yu J, Kovac J, Pastuszak AW, Lipshultz LI. Beyond the androgen receptor: the role of growth hormone secretagogues in the modern management of body composition in hypogonadal males. Transl Androl Urol. 2020 Mar;9(Suppl 2):S149-S159. doi: 10.21037/tau.2019.11.30. PMID: 32257855; PMCID: PMC7108996.
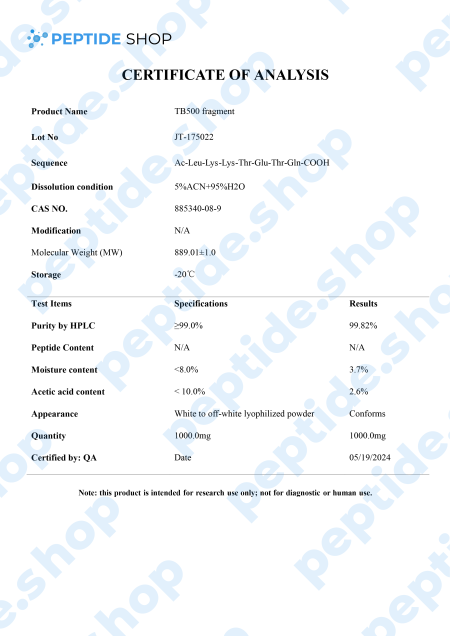
TB-500 is a 43 amino acid long synthetic peptide, analogue of thymosin beta-4. Thymosin beta-4 is a widely distributed peptide, and present in virtually all mammalian cells, which plays a pivotal role in many different processes in the body – it increases angiogenesis (formation of new blood vessels), proliferation, inhibits apoptosis (cell death) and inflammation.
Numerous animal clinical trials also showed that thymosin beta-4 can be used to indicate myocardial, liver and renal problems.
Angiogenesis:
Thymosin beta-4 promotes angiogenesis, triggers cell proliferation and migration, as well as the formation of capillary-like structures in cells. It also triggers blood perfusion (local fluid flow) by increasing capillary density.
Apoptosis:
Thymosin beta-4 inhibits apoptosis by inhibiting the transforming growth factor pathway. It also prevents nucleus pulposus (spinal disk providing shock absorption during movement) cell apoptosis and slows down cellular aging.
Inflammation:
In mouse models Tβ4 significantly dropped the number of inflammatory cells in the brains of the treated animals. It also prevented the production of proinflammatory cytokines and effectively blocked the increase of ethanol-induced inflammatory factors.
Heart Health:
Clinical data showed that thymosin beta-4 has a positive effect on both acute phase (immediately following the injury) where it preserves the ischemic myocardium, as well as in the chronic phase, in which it activates the growth of vascular cells.
After observing Tβ4’s benefits in animal models, it’s not surprising TB-500 gained so much popularity recently, since it acts as Tβ4’s synthetic analogue. Numerous clinical studies (in animal models) showed TB-500 as a potent way to improve blood vessel growth and fluid flow, accelerate wound healing, reduce oxidative stress and bind protein.
Of course, more research is needed to determine the full effects of this peptide, its safety and effectiveness in human test subjects.
Reference:
Xing Y, Ye Y, Zuo H, Li Y. Progress on the Function and Application of Thymosin β4. Front Endocrinol (Lausanne). 2021 Dec 21;12:767785. doi: 10.3389/fendo.2021.767785. PMID: 34992578; PMCID: PMC8724243.
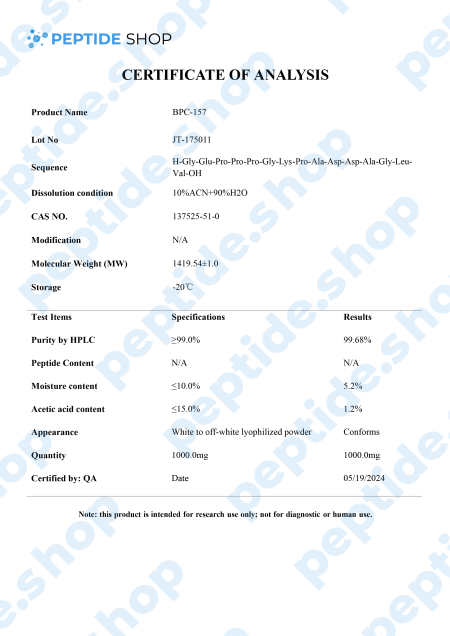
BPC-157 is a native gastric pentadecapeptide (composed of 15 amino acids), derivative of body protection compound (BPC). Being a gastric peptide allows for good oral availability and remaining stable for more than 24 hours in human gastric juice. Furthermore, there is no need for a carrier which makes this peptide unique, as compared to others that depend on it.
Clinical studies showed that BPC-157 is highly effective in both treatment and prophylaxis of various gastrointestinal lesions. This effectiveness spans to cover both acute and chronic gastric conditions, intravenous, ingastric, and even as a topical agent for deep skin burns and lesions.
Surprisingly, BPC-157 has also shown a beneficial effect on:
- Brain lesions – in one of the most recent studies researchers concluded that BPC-157 expressed a direct therapeutic effect in rat test subjects following a stroke. This peptide not only delayed neural damage, but also promoted full functional recovery.
- Behavioral disorders – medical research showed that BPC-157 counteracted catalepsy (a state of a trance or a seizure) in rat models. This research indicated a connection between BPC-157 and dopamine as well as glutamate and nitric oxide system (vital in schizophrenia therapy).
- Spinal cord injuries – A particularly interesting study showed that BPC-157 administration to rats with spinal cord injuries resulted in improvements mere 10 minutes after the initial dosage. On the contrary, untreated rats did not fully recover days, weeks, months and, in some rare cases, years following the injury.
In conclusion, we saw BPC-157 application results in a myriad of beneficial effects in various different systems within the body. Of course, many of these studies were performed on animal test subjects and we’re going to need additional ones to clarify its effect in humans and the full extent of its therapeutic potential.
References:
Vukojevic J, Milavić M, Perović D, Ilić S, Čilić AZ, Đuran N, Štrbe S, Zoričić Z, Filipčić I, Brečić P, Seiverth S, Sikirić P. Pentadecapeptide BPC 157 and the central nervous system. Neural Regen Res. 2022 Mar;17(3):482-487. doi: 10.4103/1673-5374.320969. PMID: 34380875; PMCID: PMC8504390.
Sikiric P, Hahm KB, Blagaic AB, Tvrdeic A, Pavlov KH, Petrovic A, Kokot A, Gojkovic S, Krezic I, Drmic D, Rucman R, Seiwerth S. Stable Gastric Pentadecapeptide BPC 157, Robert’s Stomach Cytoprotection/Adaptive Cytoprotection/Organoprotection, and Selye’s Stress Coping Response: Progress, Achievements, and the Future. Gut Liver. 2020 Mar 15;14(2):153-167. doi: 10.5009/gnl18490. PMID: 31158953; PMCID: PMC7096228.
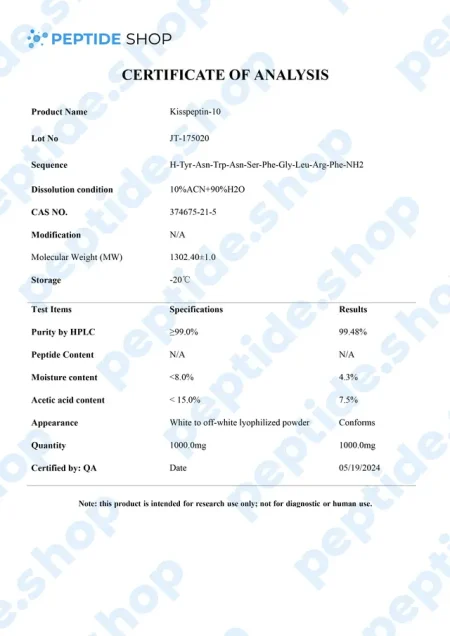
Metastin or Kisspeptin is believed to have the ability to prevent the spread of cancer or metastasis. This peptide is produced by the hypothalamus, which stimulates the release of GnRH or gonadotropin-releasing hormone, which then causes follicle-stimulating hormone and luteinizing hormone to be released from the purity gland.
Kisspeptin is used in testosterone replacement therapy instead of hCG to increase testosterone levels. That’s why it has emerged as a crucial regulator of the mammalian reproductive axis.
This peptide was first discovered in 1996 when it managed to inhibit melanoma cell lines. It belongs to a family of peptides that are derived from the KISS1/kiss1 gene structure, forming from prepro-kisspeptin, which has differential proteolysis for a common precursor.
Kisspeptin is classified as an RF or neuroactive peptide with a specific Arg-Phe-NH2 motif.
Benefits:
Kisspeptin binds to receptors in the pituitary gland, triggering a response that prompts the gland to release neurotransmitters, signaling the release of LH and FSH.
Based on numerous animal studies, scientists have managed to uncover the following benefits:
- Boosts the production of testosterone naturally
- Regulates fertility
- Increases sexual drive
- Improves immune response
- Boosts brain function
- Promotes weight loss
How does Kisspeptin work?
This peptide stimulates the release of GnRH or gonadotropin-releasing hormone. It enters the purity gland through receptor sites, causing a gland to release neurotransmitters, which later signal the releases of FH and LH. These hormones play a crucial role in the production of oestradiol and testosterone.
Kisspeptin mimics the action of hCG and clomiphene, and according to some animal studies, it can affect fertility in female and male animals.
Distribution of Kisspeptin
Kisspeptin was first found in the placenta, and later, it has been observed in the small intestine, pancreas, ovaries, and testis in mammals. The primary expression of Kisspeptin and its receptors have been shown in two big neuronal populations within the hypothalamus of rodents, the anteroventral periventricular nucleus and the arcuate nucleus.
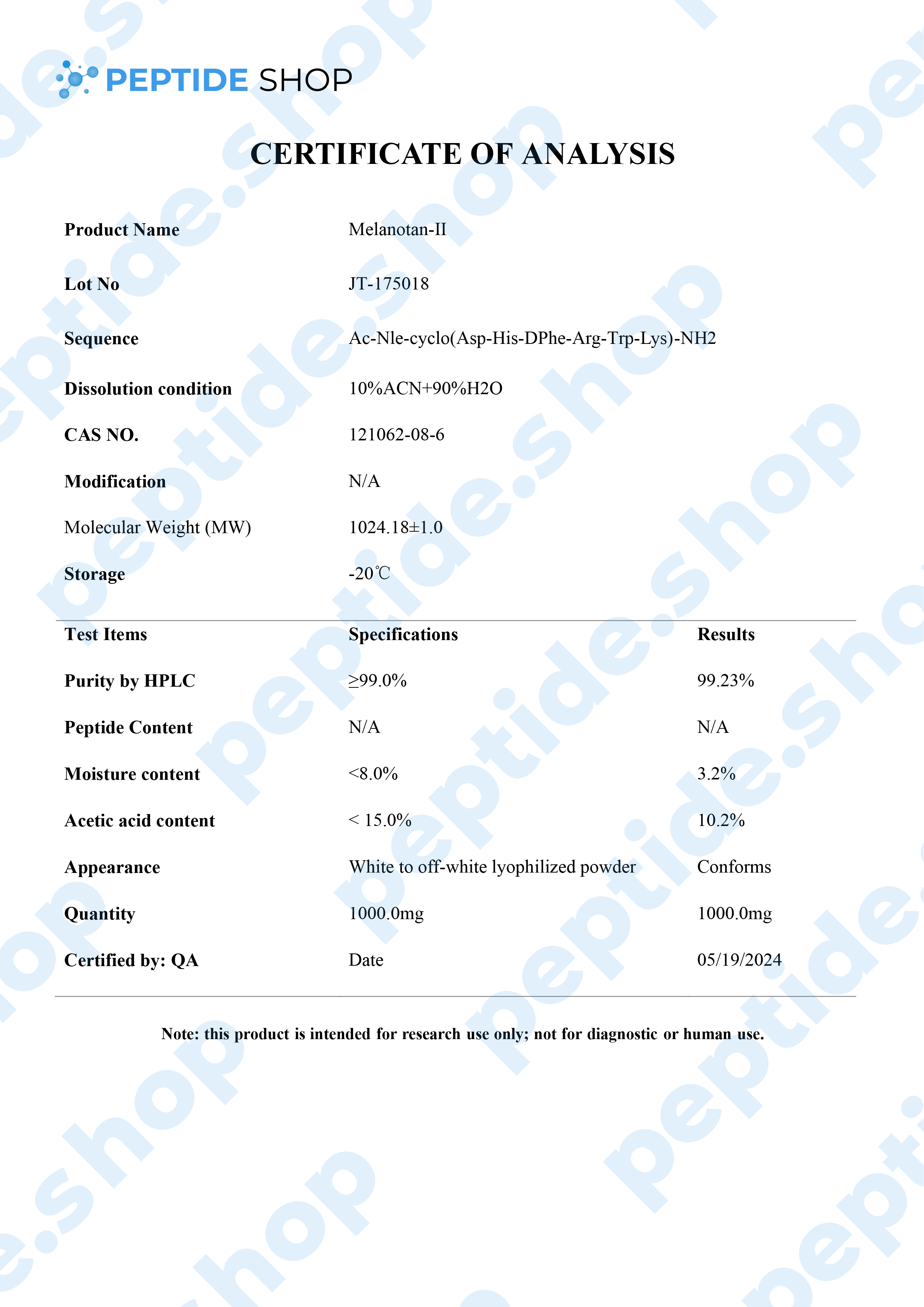
Melanotan 2 is a synthetic version of human alpha-melanocyte stimulating hormone and acts as a non selective melanocortin-receptor agonist. It was mainly developed as a sunless tanning option, but some clinical studies showed it also causes spontaneous penile erections, as well as general sexual stimulation.
Some of the other “side effects” researchers noticed were reduction in compulsive behavior, reduced glucagon production, hunger suppression and even some limited addiction control.
Melanotan 2 Effects On Autism
There is some additional recent clinical research suggesting there might be a link between Mealnotan 2 and autism. Of course, melanotan treatment cannot reverse autism, sadly, but it may act on some aspects of this disorder and make it more manageable.
In mouse model studies researchers recorded that MT2 stimulated oxytocin release, which had the potential to reduce (in some cases counteract) common ASD behaviors – behaviors that include:
- Hand clapping
- Bouncing on toes
- Body rocking
- Holding body parts in unusual positions
- Repeating vocalizations etc.
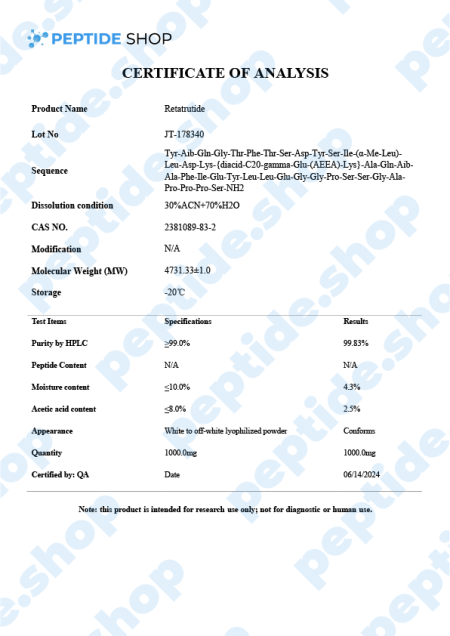
Retatrutide is one of the latest incretin mimetic drugs being tested for their weight reduction properties. Researchers use incretin mimetic agents as they work to mimic incretin hormones (gut peptides that get secreted after we eat) to allow for a better insulin secretion and hyperglycaemic control.
Even though there are numerous weight reduction agents being prescribed and used, FDA-recognized “treatment” for nonsyndromic obesity are:
- Orlistat
- Phentermine-topiramate
- Naltrexone‐bupropion
- Liraglutide and
- Semaglutide
With the exception of glucagon-like peptide 1 receptor agonists (such as semaglutide), most weight reduction agents come with a specific set of side effects such as nausea, gastrointestinal distress, abdominal pain, vomiting, diarrhea, or a general weight decrease that does not cross the 15% mark. Some of these medications even come with a warning against using them if a patient is suffering from a kidney, heart, thyroid or any related problems.
This is why scientists are turning more and more to retatrutide, because this incretin mimetic decreases appetite, promotes the feeling of fullness and slows down the process of gastric emptying, but does all that while presenting with minimal side effects and increased weight loss effectiveness, compared to liraglutide or semaglutide.
Retatrutide Mechanism of Action
Retatrutide exerts a powerful glucagon-receptor agonistic effect. When we compare it to human glucagon and glucagon-like peptide 1 (GLP-1), it exhibits:
- Reduced potency on the GCGR (human glucagon receptor) and GLP-1R (glucagon-like peptide-1 receptor)
- Enhanced potency at the human GIPR (gastric inhibitory polypeptide receptor)
When talking about in vitro experiments, retatrutide demonstrated similar efficacy in evoking glucose production within hepatocytes and inducing lipolysis. Also, retatrutide showed a favorable half-life of 6 days, enabling it a weekly dosage regimen (should the drug get FDA approved).
Another double-blind study conducted in the US on obese patients showed promising results. 338 adults in total were involved, split into 6 cohorts (depending on the weekly dosage), up against a placebo group. Retatritude group experienced the following results:
- 1mg group – after 24 weeks this group experienced weight loss of 7.2%, and after 48 weeks a total loss of 8.7%
- 4mg group (commencing with 2mg) – after 24 weeks this group experienced a weight loss of 11.8%, and after 48 weeks a total loss of 16.3%
- 4mg group (commencing with 4mg) – after 24 weeks this group experienced a weight loss of 13.9%, and after 48 weeks a total loss of 17.8%
- 8mg group (commencing with 2mg) – after 24 weeks this group experienced a weight loss of 16.7%, and after 48 weeks a total loss of 21.7%
- 8mg group (commencing with 4mg) – after 24 weeks this group experienced a weight loss of 17.9%, and after 48 weeks a total loss of 23.9%
- 12mg group (commencing with 2mg) – after 24 weeks this group experienced a weight loss of 17.5%, and after 48 weeks a total loss of 24.2%
In contrast, the placebo group only experienced a 1.6% weight loss after 24 weeks and 2.1% after 48. Findings also showed that adverse effects related to retratitude treatment (such as nausea, vomiting, diarrhea and constipation), were mild and depended on the dosage.
In conclusion, this peptide showed promising results in addressing obesity in both in vitro and human test subject studies. However, test samples were relatively small, and we still need more research and clinical trials to show the effectiveness of retratutide as an effective, long-term way of managing weight.
Reference:
Naeem M, Imran L, Banatwala UESS. Unleashing the power of retatrutide: A possible triumph over obesity and overweight: A correspondence. Health Sci Rep. 2024 Feb 5;7(2):e1864. doi: 10.1002/hsr2.1864. PMID: 38323122; PMCID: PMC10844714.
Semax is an interesting nootropic that can capture the attention of researchers, health professionals, and enthusiasts alike. It was originally developed in Russia back in the 1980s and 1990s. Nowadays, Semax is believed to enhance a range of cognitive and neuroprotective properties.
Many know this peptide by its trade name, such as Semaxum, and it has been extensively researched by institutions such as the Institute of Molecular Genetics of the Russian Academy of Science.
What is Semax?
Semax is a peptide best known for its neurogenic, neuroprotective, and nootropic properties. It was developed based on the molecular structure of ACTH or adrenocorticotropic hormone. Preliminary studies in small-scale human trials, animals, and cells indicate potential benefits of Semax use.
In the U.S. and multiple countries around the world, this component is the basis for a number of medicines that are used in clinical practices for the treatment of optic nerve atrophy, dys-circulatory encephalopathy, and ischemic brain stroke.
Potential benefits
Some of the benefits Semax may include:
- Increased short-term memory and attention
- Better non-proliferative diabetic neuropathy
- Can assist during recovery from hypoxia/stroke
- Can help with glaucoma optic neuropathy
- May act as an analgesic
- It may help in treating ADHD
- It may help protect the brain from various types of damage and stress
How Does Semax Work?
Semax increases BDNF or brain-derived neurotrophic factor. BDNF is one of the most active neurotrophins, which help control and stimulate neurogenesis, creating new neurons in the brain. This component helps support neuroplasticity, allowing nerve cells in the brain to adapt to new situations and recover from injury.
Additionally, BDNF encourages the survival of existing neurons and supports the growth, creation, and regeneration of new synapses and neurons.
This nootropic also helps minimize the breakdown of enkephalins. Enkephalins are mostly involved in reducing inflammation, decreasing pain, boosting immune cell activity, and preventing cancer cell growth.
They also play a role in pain, emotional behavior, learning, and memory. Balanced enkephalins are necessary to maintain normal brain function. Therefore, researchers are led to believe that Semax may potentially relieve pain. However, more research is needed to confirm the preliminary findings.
References:
Medvedeva EV, Dmitrieva VG, Povarova OV, Limborska SA, Skvortsova VI, Myasoedov NF, Dergunova LV. The peptide semax affects the expression of genes related to the immune and vascular systems in rat brain focal ischemia: genome-wide transcriptional analysis. BMC Genomics. 2014 Mar 24;15:228. doi: 10.1186/1471-2164-15-228. PMID: 24661604; PMCID: PMC3987924.
https://www.sciencedirect.com/science/article/abs/pii/S0306987706005391
Gusev, E. & Martinov, Michail & Kostenko, E. & Petrova, Lyudmila & Bobyreva, S.. (2018). The efficacy of semax in the tretament of patients at different stages of ischemic stroke. Zhurnal nevrologii i psikhiatrii im. S.S. Korsakova. 118. 61. 10.17116/jnevro20181183261-68.
Cagrisema 10mg is one of the latest PeptideShop’s peptide blends, which combines cagrilintide and semaglutide. Each vial contains a combination of lyophilized cagrilintide and semaglutide, 5mg each, tested for purity and strength, and suitable for lab experiments and chemical trials.
Semaglutide is a GLP-1 receptor agonist. GLP-1 is one of the main gut peptide hormones in humans, which plays a role in many different mechanisms within the body from insulin secretion, glucagon release, gastric emptying, appetite and energy intake.
And cagrilintdide is an amylin analogue co-secreted with insulin, which plays a role in glycemic control and gastric emptying.
Researchers hypothesize that this combination profoundly influences receptor changes in the brain, insulin secretion, appetite regulation and glucagon secretion.
Though this combination has not yet been fully tested, we have data from a study on the effects of Cagrisema on patients with type 2 diabetes.This was a 32-week, double-blind study across 17 sites in the USA, conducted on 92 individuals in total.
Final results showed that cagrisema resulted in clinically relevant improvements in glycaemic control (significant change in HbA1c) and, more importantly, a greater weight loss versus semaglutide as well as cagrilintide.
References:
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)01163-7/abstract
Cerebrolysin is a porcine-derived peptide preparation, with a low molecular mass and a variety of research applications. An interesting thing about cerebrolysin is that its preparation contains nerve growth factors, BDFN (brain derived neurotrophic factor), Ciliary nerve growth factor, p-21 and orexin. This means that cerebrolysin contains molecules with:
- Pharmacodynamic properties – it expresses biochemical, physiological and molecular effects within the body.
- Neurotrophic properties – it aids in growing, repair and neuron maintenance
- Neuroprotective properties – it strengthens the neural pathways as well as the neurons themselves and improves synaptic plasticity.
Cerebrolysin Chemical Structure
It’s a bit difficult talking about cerebrolysin as it does not have a single chemical structure, rather, it’s a combination of several different peptides. As such, cerebrolysin is actually labeled an “orphan drug” by the FDA. What this means is it did show promise in disease treatment and prevention (in clinical trials), but only in orphan diseases. Orphan disease is one that affects fewer than 200,000 people in the US. This is why this peptide mix is not a profitable venture for further, independent studies, and research is only possible with financial help from the government.
But in the particular case of cerebrolysin, the government did, in fact, intervene, due to its potential in dealing with dementia and because of its neuroprotective capabilities.
This peptide mix was first developed in Austria, back in 1940 and has since become a vital medicine in Asia, especially Russia and China. Research studies showed that it has the ability to cross the blood-brain barrier and express its pharmacodynamic effects on both brain and the spinal cord.
But, what does it do?
Since cerebrolysin is not a single chemical compound, as we already said, we can look at it, and its effects, through the components its made of:
- Brain-Derived Neurotrophic Factor (BDNF) – this is a protein found in our central nervous system, shown to have a profound effect on neuron growth, synapse growth and health. Research in depression and Alcheimer’s disease revealed disrupted BDNF pattern expression.
- Glial Cell Line-Derived Neurotrophic Factor (GCNF) – this is an exceptionally important, naturally occurring peptide that promotes neuron survival, decreases the loss of neurons and, as some studies have pointed out, has the potential to aid the prevention of Parkinson’s and ALS.
- Ciliary Neurotrophic Factor (CNTF) – this is an interesting hormone affecting the growth of certain neural cells and has been a subject of numerous trials aimed at treating ALS.
- Nerve Growth Factor (NGF) – this is a particularly important peptide which regulates the growth, survival and neuron increase in number. Some studies showed NGF is one of the important factors in programmed cell death and regulation of certain immune system mechanisms.
Looking at these effects, it’s not hard to imagine why scientists used these molecules – to try and merge all these positive effects into one single mix. But were they successful? We actually have some clinical trials atesting to these possibilities:
- Cerebrolysin and Dementia – a study done in 2012 evaluated the outcomes of cerebrolysin therapy in patients with Alcheimer’s over the course of a 12 week treatment period. The study showed significant improvements in cognitive functions and the symptoms of dementia lasting up to several months after the study was finished.
- Cerebrolysin and Parkinson’s – we know that the onset of Parkinson’s disease has to do with the protection of dopamine producing neurons. The loss of these neurons is what leads to motor deficits characteristic for this disease. Unfortunately, we haven’t had human trials, but the one on mice showed that cerebrolysin administration protected these neurons and also lowered certain hormones responsible for Parkinson’s disease worsening.
- Stroke and Brain Injuries – though we don’t have many studies dealing with this topic, some small-scale ones showed cerebrolysin as being not only safe to use, but also helpful in recovery rate if given within 72 hours. Research in infants with brain injury induced communication delay also showed cerebrolysin improved therapeutic outcomes.
Even though these positive effects may seem overwhelming, leaving you wondering why cerebrolysin is not a mainstream, FDA-approved drug still, it’s important to note most of these findings were done on a limited sample (and mostly on animal studies). We have yet to see its effects in large-scale studies and, more importantly, see the long-term benefits of cerebrolysin therapy. Even though the initial results seem promising, we need to be patient and wait for more information before the final verdict.
References:
https://gsrs.ncats.nih.gov/ginas/app/beta/substances/37KZM6S21G%20
Allegri RF, Guekht A. Cerebrolysin improves symptoms and delays progression in patients with Alzheimer’s disease and vascular dementia. Drugs Today (Barc). 2012 Apr;48 Suppl A:25-41. doi: 10.1358/dot.2012.48(Suppl.A).1739721. PMID: 22514793.
Plosker GL, Gauthier S. Cerebrolysin: a review of its use in dementia. Drugs Aging. 2009;26(11):893-915. doi: 10.2165/11203320-000000000-00000. PMID: 19848437.
Masliah E, Armasolo F, Veinbergs I, Mallory M, Samuel W. Cerebrolysin ameliorates performance deficits, and neuronal damage in apolipoprotein E-deficient mice. Pharmacol Biochem Behav. 1999 Feb;62(2):239-45. doi: 10.1016/s0091-3057(98)00144-0. PMID: 9972690.
Ozkizilcik A, Sharma A, Lafuente JV, Muresanu DF, Castellani RJ, Nozari A, Tian ZR, Mössler H, Sharma HS. Nanodelivery of cerebrolysin reduces pathophysiology of Parkinson’s disease. Prog Brain Res. 2019;245:201-246. doi: 10.1016/bs.pbr.2019.03.014. Epub 2019 Apr 2. PMID: 30961868.
Ozkizilcik A, Sharma A, Lafuente JV, Muresanu DF, Castellani RJ, Nozari A, Tian ZR, Mössler H, Sharma HS. Nanodelivery of cerebrolysin reduces pathophysiology of Parkinson’s disease. Prog Brain Res. 2019;245:201-246. doi: 10.1016/bs.pbr.2019.03.014. Epub 2019 Apr 2. PMID: 30961868.
Hassanein SM, Deifalla SM, El-Houssinie M, Mokbel SA. Safety and Efficacy of Cerebrolysin in Infants with Communication Defects due to Severe Perinatal Brain Insult: A Randomized Controlled Clinical Trial. J Clin Neurol. 2016 Jan;12(1):79-84. doi: 10.3988/jcn.2016.12.1.79. Epub 2015 Sep 11. PMID: 26365023; PMCID: PMC4712290.
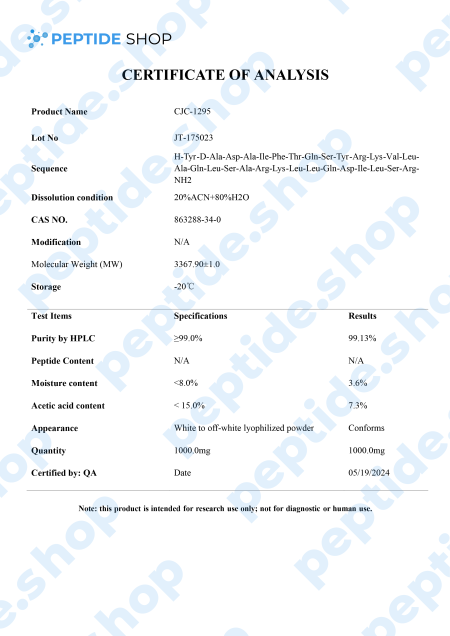
CJC-1295 is a synthetic analogue of growth hormone-releasing hormone (GHRH) and growth hormone secretagogue (GHS) developed by ConjuChem Biotechnologies.
GHRH is a 44-amino acid long peptide which our hypothalamus synthesizes and in the pituitary gland, it binds to the growth hormone (GH) receptors, resulting in the release, regulation and pulsatile secretion of GH.
We already talked about GH therapy as being FDA approved in conditions such as GH deficiency, Turner syndrome, Prader-Willi syndrome, idiopathic short stature etc. Recombinant human GH treatment is generally performed as one daily, subcutaneous injection which elevates the levels of GH serum in the blood.
One of the major problems with this approach to treatment is that the efficacy of GH therapy is hard to determine due to the lack of biological serum biomarkers. Currently, most facilities are using levels of IGF-1 and IGFBP-3 to monitor the efficacy of this therapy but these levels may vary wildly (due to growth velocity, glucose tolerance, insulin levels etc.).
Furthermore, GH abuse extended across multiple sports disciplines, making it even harder to suppress and put this problem under control.
Researchers hope to solve this problem by employing new biomarkers of GH action and secretion. One such way is to employ newly developed molecules, such as CJC-1295, shown to increase both GH and IGF-1 levels in the blood, without affecting the pulsatility of GH secretion. CJC-1295 also has a prolonged half-life of 8 to 10 days, due to its ability to bind to the endogenous serum albumin.
So, what scientists are hoping to achieve with CJC-1295 is both a safe way of promoting GH secretion as well as making it measurable in a laboratory setting. There were numerous studies tackling this issue, but we still need further testing to confirm these preliminary findings.
Reference:
Sackmann-Sala L, Ding J, Frohman LA, Kopchick JJ. Activation of the GH/IGF-1 axis by CJC-1295, a long-acting GHRH analog, results in serum protein profile changes in normal adult subjects. Growth Horm IGF Res. 2009 Dec;19(6):471-7. doi: 10.1016/j.ghir.2009.03.001. Epub 2009 Apr 21. PMID: 19386527; PMCID: PMC2787983.


 Anti Aging
Anti Aging Hair Growth
Hair Growth Muscle Growth
Muscle Growth Peptide Blends
Peptide Blends Peptide Supplies
Peptide Supplies Peptides
Peptides Skin
Skin Testosterone
Testosterone Weight Loss
Weight Loss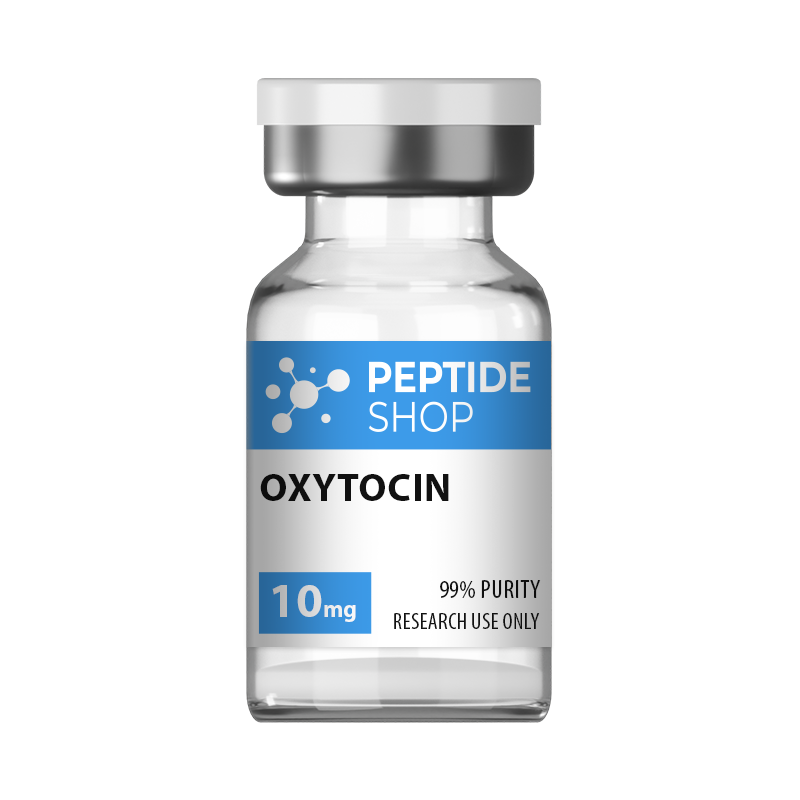








Reviews
There are no reviews yet.